Bedaquiline – Side Effects & Patent Rejection
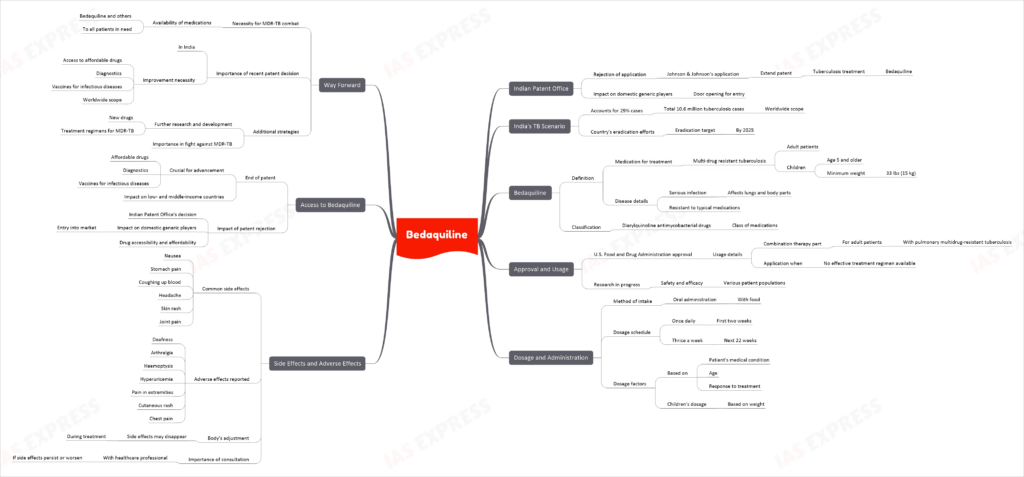
Recently, the Indian Patent Office rejected Johnson & Johnson’s application to extend its patent on the tuberculosis treatment, bedaquiline, opening the door for domestic generic players.
As India accounts for nearly 29% of the 10.6 million tuberculosis cases worldwide, this decision is significant in the country’s efforts to eradicate tuberculosis by 2025.
What is Bedaquiline?
Bedaquiline is a medication used along with at least three other medications to treat multi-drug resistant tuberculosis (MDR-TB) in adults and children aged 5 years and older who weigh at least 33 lbs (15 kg). MDR-TB is a serious infection that affects the lungs and other parts of the body and cannot be treated with other medications typically used for the condition. Bedaquiline belongs to a class of medications called diarylquinoline antimycobacterial drugs.
Approval and Usage
Bedaquiline is approved by the U.S. Food and Drug Administration (FDA) for use as part of a combination therapy in adults with pulmonary multidrug-resistant tuberculosis (MDR-TB) when an effective treatment regimen cannot otherwise be provided. The safety and efficacy of bedaquiline in different patient populations are still being studied.
Dosage and Administration
Bedaquiline is taken orally with food, usually once daily for the first two weeks, followed by three times a week for the next 22 weeks. The dosage is based on the patient’s medical condition, age, and response to treatment. Children’s dosage is also based on weight.
Side Effects and Adverse Effects
Some common side effects of bedaquiline include nausea, stomach pain, coughing up blood, headache, skin rash, and joint pain. Other adverse effects reported include deafness, arthralgia, haemoptysis, hyperuricemia, pain in the extremities, cutaneous rash, and chest pain. These side effects may go away during treatment as the body adjusts to the medication. It is important to consult a healthcare professional if side effects persist or worsen.
Access to Bedaquiline
The end of the bedaquiline patent is a crucial development for moving forward with affordable drugs, diagnostics, and vaccines for infectious diseases in low- and middle-income countries. With the Indian Patent Office’s rejection of Johnson & Johnson’s application to extend its patent on bedaquiline, domestic generic players can now enter the market, making the drug more accessible and affordable.
Way Forward
To effectively combat MDR-TB, it is essential to ensure that bedaquiline and other life-saving medications are made available to all patients who need them. The recent patent decision in India is a step in the right direction, but continued efforts are needed to improve access to affordable drugs, diagnostics, and vaccines for infectious diseases worldwide. Additionally, further research and development of new drugs and treatment regimens for MDR-TB are crucial in the ongoing fight against this devastating disease.
If you like this post, please share your feedback in the comments section below so that we will upload more posts like this.