R21/Matrix-M vaccine
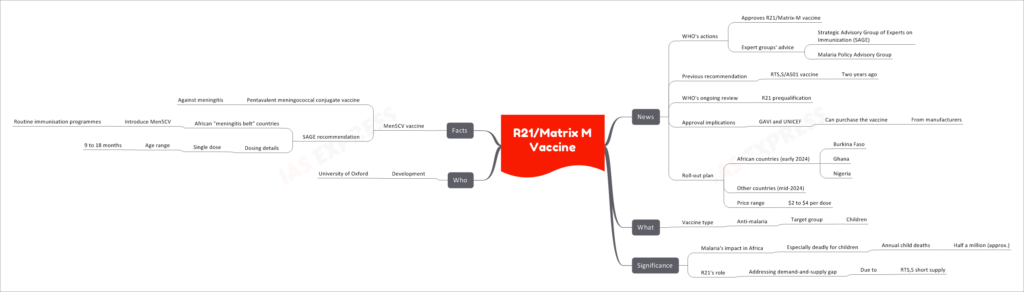
The World Health Organization (WHO) has taken a significant step by approving the R21/Matrix-M vaccine as part of its ongoing efforts to combat malaria. This move comes following the recommendations of expert groups, including the Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Group.
This topic of “R21/Matrix-M vaccine” is important from the perspective of the UPSC IAS Examination, which falls under General Studies Portion.
Previous Recommendation: RTS,S/AS01 Vaccine
Two years ago, the WHO recommended the RTS,S/AS01 vaccine as a crucial tool in the fight against malaria.
WHO’s Ongoing Review: R21 Prequalification
The WHO is currently conducting a review for the prequalification of the R21 vaccine.
Approval Implications
With the WHO’s approval, organizations such as GAVI (The Vaccine Alliance) and UNICEF can now purchase the R21/Matrix-M vaccine from manufacturers to facilitate its distribution.
Roll-Out Plan
The roll-out plan for the R21/Matrix-M vaccine is as follows:
- African Countries (Early 2024): Burkina Faso, Ghana, and Nigeria are among the first African nations slated to receive the vaccine.
- Other Countries (Mid-2024): Additional countries will follow suit.
- Price Range: The vaccine is expected to be available at a price ranging from $2 to $4 per dose.
What is R21/Matrix-M?
Vaccine Type
The R21/Matrix-M vaccine is an anti-malaria vaccine specifically designed for children.
Significance
Malaria’s Impact in Africa
Malaria remains a significant health threat in Africa, particularly deadly for children. Annually, it results in the loss of approximately half a million young lives.
R21’s Role
The approval of the R21/Matrix-M vaccine is expected to address the demand-and-supply gap that emerged due to a shortage of the RTS,S vaccine.
Who Developed R21/Matrix-M?
The R21/Matrix-M vaccine was developed by the University of Oxford.
Facts: Men5CV Vaccine
In addition to the R21/Matrix-M vaccine, the WHO has also recommended the Men5CV vaccine, which is a pentavalent meningococcal conjugate vaccine designed to protect against meningitis. The SAGE recommendation calls for African countries within the “meningitis belt” to introduce Men5CV into their routine immunization programs. This vaccine is administered as a single dose for children aged 9 to 18 months in these regions.

