Convalescent Plasma Therapy for COVID-19: How does it Work & Challenges
Recently, a private hospital in Delhi became the first hospital in India to use convalescent plasma therapy to treat two coronavirus patients who were in the ICU, despite the lack of approval from ICMR and DCGI. This was based on the provision that allows for “Off Label Use” in the case of critically ill patients of an already approved drug to be used for a new purpose. The Indian Council of Medical Research (ICMR) had sought the participation of researchers for conducting a clinical trial of convalescent plasma therapy to treat critically ill coronavirus patients and floated a protocol for it. Several clinical trials of plasma therapy to combat coronavirus have proven treatment to be effective. This treatment still requires may more studies and experiments to prove efficiency.
This topic of “Convalescent Plasma Therapy for COVID-19: How does it Work & Challenges” is important from the perspective of the UPSC IAS Examination, which falls under General Studies Portion.
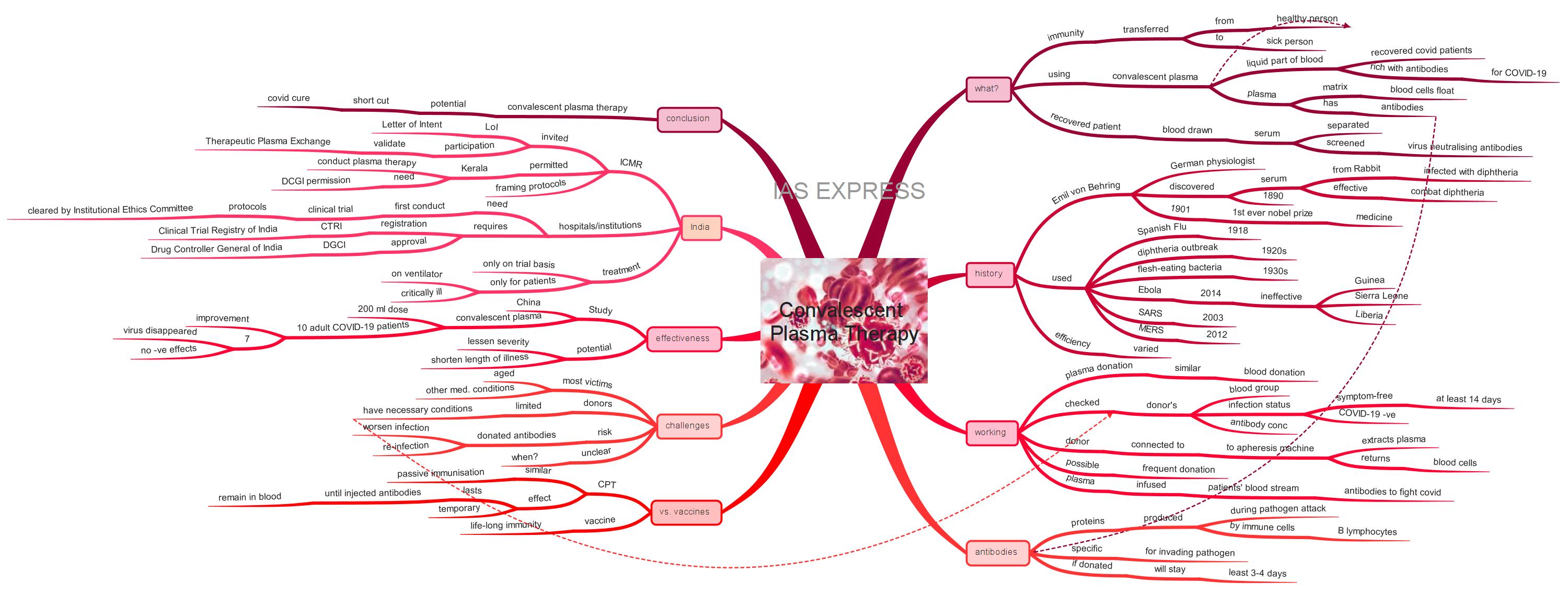
What is convalescent plasma therapy?
- It is a treatment wherein the immunity is transferred from a healthy person to a sick person using convalescent plasma.
- Convalescent plasma refers to the liquid part of the blood from the recovered COVID-19 patients, which is rich with antibodies that are capable of fighting the coronavirus.
- Plasma is a matrix on which blood cells float. It also houses crucial proteins of immunity known as antibodies.
- When attacked by a pathogen like a coronavirus, the immune system produces antibodies to fight the infection.
- Antibodies are a particular type of protein secreted by immune cells called B lymphocytes when they encounter a pathogen such as COVID-19.
- The immune system designs antibodies that are highly specific to each invading pathogen. A particular antibody and its partner virus are made for each other
- If the infected person can produce sufficient antibodies that can counter COVID-19, he/she can recover from the coronavirus infection.
- It is found that immunity develops early in asymptomatic or persons with mild symptoms.
- On the other hand, it develops much later for severe and critically-ill COVID-19 patients.
- Through the convalescent plasma therapy, blood is drawn from a COVID-19 recovered person and the serum is separated and screened for virus neutralizing antibodies to be used on the severe and critically ill patients who have limited antibodies to combat COVID-19.
When was it previously used?
- In 1890, Emil von Behring, a German physiologist, discovered that the serum obtained from a rabbit infected with diphtheria was effective in preventing the diphtheria infection.
- Behring was awarded the first-ever Nobel prize for medicine in 1901.
- Similar treatments were used during several outbreaks in the past, including the Spanish Flu pandemic of 1918, the diphtheria outbreak in the 1920s, a flesh-eating bacteria epidemic in the 1930s.
- Back then, the convalescent serum therapy was less effective and had substantial side effects.
- It took many years before the antibody fraction could be separated.
- Still, the unintended antibodies and impurities caused side effects.
- More recently, it was experimented for Ebola and other coronavirus diseases like SARS in 2003 and MERS in 2012, with varied efficiency.
- The convalescent plasma that was used to treat Ebola in 2014 in Guinea, Sierra Leone and Liberia found to be ineffective.
- Currently, with the improvement of extraction and screening techniques, the method appears much more safe and effective than ever before.
How does plasma therapy work?
- The process of plasma donation is similar to that of blood donation.
- The donors’ blood group, infection status and antibody concentration is initially established.
- Then, these donors are connected to the apheresis machine through a needle in one arm.
- As the blood flows through it, the machine extracts the yellowish, anti-body rich plasma, while returning red and white blood cells back to the donor.
- Unlike regular blood donations, plasma can be donated more frequently, as often as twice a week.
- The collected plasma, which is enough for several patients, is then chilled to minus 18 C and either stored or dispatched where it is needed.
- The collected plasma is then infused into the bloodstream of the patients, helping them by providing necessary antibodies to combat the pathogen.
How long will the antibodies remain in the recipient?
- After the antibody serum is given, it will stay with the recipient for at least 3-4 days.
- During this period, the recipient will recover.
- Research reports from the United States and China indicate that the beneficial effect of transfusion plasma is obtained in the first 3 to 4 days and not later.
How is it different from vaccination?
- The therapy is similar to passive immunisation.
- When a vaccine is administered, the immune system produces antibodies.
- Thus, on a later date, when the vaccinated person is infected by that pathogen, the immune system releases the antibodies and neutralises the infection.
- Vaccination provides lifelong immunity.
- In the case of passive antibody therapy, the effect lasts only until the injected antibodies remain in the bloodstream.
- It gives only temporary protection against the pathogen
What are the challenges?
- In diseases like COVID-19, most of the victims are aged, suffering from other medical conditions like hypertension, diabetes etc.
- Not all survivors can volunteer to donate plasma. They must have high concentrations of antibodies against the virus.
- They must have been symptom-free for at least 14 days and test negative for COVID-19.
- Thus, very few donors are available based on these conditions.
- Experts are warning about the antibody-mediated enhancement of infection, where the donated antibodies can make the infection worse or bring in the potential for re-infection.
- It is not clear when the treatment can be given for it to be effective.
- Early research suggested that convalescent plasma might be most effective when given to people before they get sick or early in the course of the disease.
How effective is convalescent plasma therapy?
- A study in China found that the therapy is effective, though on a small sample size, in treating the COVID-19 patients.
- In the trial, a 200 ml dose of convalescent plasma was administered to 10 adult COVID-19 patients with severe symptoms.
- The patients witnessed significant improvement with the disappearance of the virus reported among seven patients without any adverse side effects.
- Based on the assessment of previous experiences with respiratory viruses and data acquired from China, it is found that the therapy has the potential to lessen the severity or shorten the length of illness caused by the COVID-19.
- Globally, nearly 5 lakh coronavirus cases have recovered completely.
- Therefore, there is a possibility of a sufficient supply of antibodies to treat critically ill patients if the therapy is proven to be effective.
Is India considering the use of Convalescent Plasma Therapy?
- The Indian Council of Medical Research (ICMR) has invited a Letter of Intent (LoI) for participation in studies to validate Therapeutic Plasma Exchange (TPE), an experimental procedure to treat critically ill COVID-19 patients.
- Hospitals and institutions that are planning to provide convalescent plasma therapy must first conduct a clinical trial with protocols cleared by the Institutional Ethics Committee (EIC).
- They must also be registered with the Clinical Trial Registry of India (CTRI) and get approval from the Drug Controller General of India (DCGI) before initiating the trial.
- Kerala had initiated research and protocols for plasma therapy even before the US Food and Drug Administration (FDA) approved it on 3rd
- ICMR has permitted Kerala to conduct plasma therapy, making it the first state to be given a nod by India’s apex medical research organisation.
- Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST) under the Department of Science and Technology was given a go-ahead by the ICMR on 11th
- However, the approval from DCGI is pending as there are rules like blood donors should not have travelled abroad in the past three months.
- The ICMR is currently framing protocol for infusing blood plasma from people who have recovered from COVID-19 into critically ill patients.
- Currently, this treatment is allowed only on a trial basis for patients with a serious condition or on the ventilator.
Conclusion:
With a growing number of recovered cases, the convalescent plasma therapy has the potential to provide a much-needed short cut in finding the cure for the COVID-19.
Practice question for mains:
What is convalescent plasma therapy? How can it help combat coronavirus pandemic? (250 words)