Ammonia Gas: Properties, Applications, Health Effects
Summary
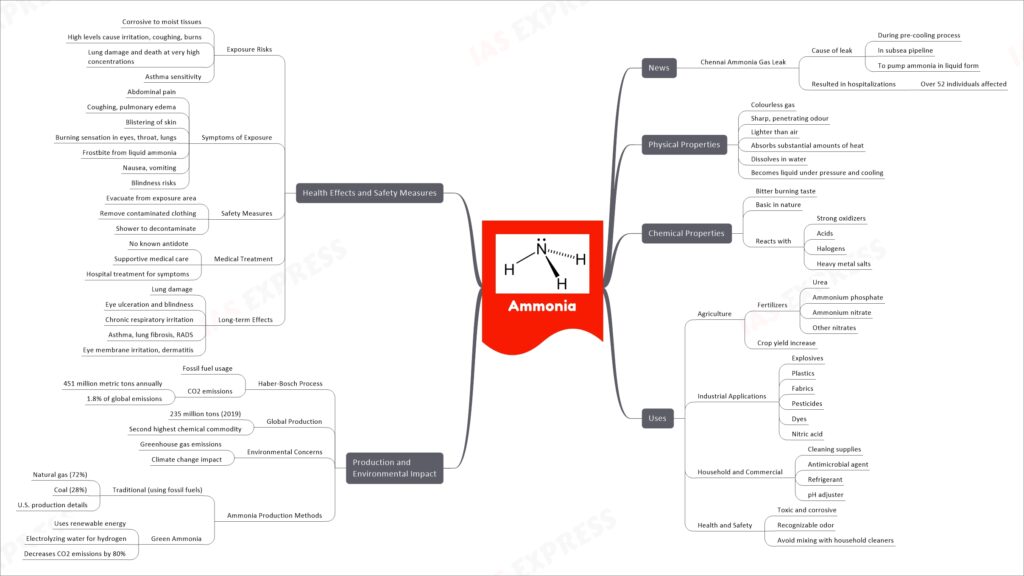
Ammonia is a colorless gas, lighter than air, with a sharp odor, and it dissolves in water. It’s widely used in agriculture as a fertilizer to increase crop yield and in various industries to produce explosives, plastics, and other chemicals. The production of ammonia, mainly through the Haber-Bosch process using fossil fuels, contributes significantly to CO2 emissions and global warming. However, green ammonia production methods using renewable energy sources are emerging to reduce these environmental impacts. Exposure to ammonia can be harmful, causing skin and lung irritation, coughing, and even blindness at high concentrations. It’s essential to evacuate the exposed area and decontaminate by removing clothes and showering. There is no antidote for ammonia exposure, so medical treatment focuses on symptom management. Long-term exposure can lead to serious health conditions like lung damage, chronic respiratory irritation, and eye problems.
Article
Introduction:
Ammonia is a versatile chemical compound with a wide range of applications across various industries. In recent news, a tragic incident in Chennai involving an ammonia gas leak has highlighted the importance of understanding the properties, uses, production methods, and safety measures associated with this compound. This article will delve into the world of ammonia, shedding light on its physical and chemical properties, uses, production processes, environmental impact, health effects, and safety precautions.
Physical Properties of Ammonia:
- Colorless Gas: Ammonia is a colorless gas, making it challenging to detect without specialized equipment.
- Sharp Odor: It possesses a sharp, penetrating odor that is easily recognizable even at low concentrations.
- Lighter than Air: Ammonia is lighter than air, causing it to rise when released into the atmosphere.
- Heat Absorption: It has the remarkable ability to absorb substantial amounts of heat from its surroundings.
- Solubility in Water: Ammonia readily dissolves in water, forming a highly alkaline solution.
- Liquid Form Under Pressure: When subjected to pressure and cooling, ammonia transforms into a liquid state.
Chemical Properties of Ammonia:
- Bitter Taste: Ammonia has a bitter burning taste, which can be detected in high concentrations.
- Basic Nature: It is a basic substance with a pH greater than 7.
- Reactivity: Ammonia reacts vigorously with various substances, including strong oxidizers, acids, halogens, and heavy metal salts.
Uses of Ammonia:
- Agriculture: Ammonia is a crucial component of fertilizers, including urea, ammonium phosphate, ammonium nitrate, and other nitrates, which contribute to increased crop yields.
- Industrial Applications: It finds applications in explosives, plastics, fabrics, pesticides, dyes, and the production of nitric acid.
- Household and Commercial: Ammonia is used in cleaning supplies, as an antimicrobial agent, refrigerant, and pH adjuster.
- Health and Safety: It serves as a toxic and corrosive substance with a recognizable odor, and it is essential to avoid mixing it with household cleaners.
Production and Environmental Impact:
- Haber-Bosch Process: The traditional method of producing ammonia involves fossil fuels, resulting in significant CO2 emissions, contributing to climate change.
- Global Production: In 2019, global production reached 235 million tons, making ammonia the second highest chemical commodity.
- Environmental Concerns: The production and usage of ammonia lead to greenhouse gas emissions and have a notable impact on climate change.
- Ammonia Production Methods: The emergence of “Green Ammonia” involves using renewable energy and electrolyzing water for hydrogen, reducing CO2 emissions by up to 80%.
Health Effects and Safety Measures:
- Exposure Risks: Ammonia is corrosive to moist tissues and can cause irritation, coughing, burns, lung damage, and even death at very high concentrations. It can also trigger asthma sensitivity.
- Symptoms of Exposure: Exposure may lead to abdominal pain, coughing, pulmonary edema, skin blistering, eye, throat, and lung irritation, frostbite, nausea, vomiting, and blindness risks.
- Safety Measures: In case of exposure, it is crucial to evacuate the area, remove contaminated clothing, and shower to decontaminate. Medical treatment primarily focuses on supportive care.
- Long-term Effects: Long-term exposure to ammonia can result in lung damage, eye ulceration and blindness, chronic respiratory irritation, asthma, lung fibrosis, and reactive airway dysfunction syndrome (RADS).
Conclusion:
Ammonia’s significance in various industries and its potential hazards underscore the need for strict safety protocols and environmentally friendly production methods. Understanding its properties, uses, and health effects is essential for both professionals working with ammonia and the general public to prevent accidents and mitigate risks associated with this versatile compound.
If you like this post, please share your feedback in the comments section below so that we will upload more posts like this.