INTRODUCTION
Proteins are polymers of amino acids and are the most abundant organic molecules of the living system constituting about 50% of the cellular dry weight.
AMINO ACIDS:
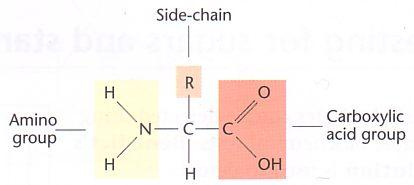
Amino acids are organic compounds containing two functional groups i.e., amino (-NH2) group and carboxyl (-COOH) group along with a side chain specific to each amino acid.
STRUCTURE OF AMINO ACIDS:
Chemically, an amino acid consists of four components:
- Carboxylic group (-COOH), which is basic in nature.
- Amino group (-NH2), which is acidic in nature.
- R side chain which is specific for each amino acid and accounts for the different types of amino acids.
- Hydrogen atom linked to the central carbon atom.
If both the carboxyl group and amino groups are attached to the same carbon, the amino acid is termed as α amino acid.
- There are almost 400 identified amino acids occurring in nature however, only 20 are involved in protein formation in living organisms.
- These 20 proteins arrange in various combinations to produce different types of proteins in living organism.
PEPTIDE LINKAGE:
The adjacent amino acids in a polypeptide protein chain are linked together via peptide bonds.
DEFINITION:
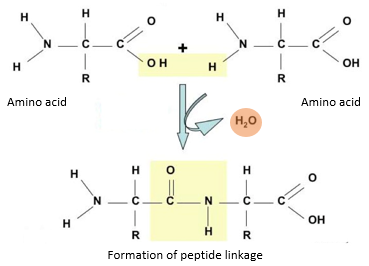
Peptide linkage is the bond formed by the reaction of the amino group of one amino acid and the carboxyl group of another resulting in the release of water molecule (condensation).
EXPLANATION:
- During the formation of peptide bond, one amino acid loses hydroxyl (-OH) group from its carboxylic acid group, while the other loses a hydrogen atom from its amino group.
- This leaves a carbon atom of the first amino acid free to bond with the nitrogen atom of the second.
- Thus, a bond is formed between the carbon and nitrogen atoms (-C-N-) of successive amino acids leading to the formation of a dipeptide. This bond is termed as a peptide bond.
- The hydroxyl group and hydrogen lost during the bond formation combines to form a water molecule.
- Therefore, peptide linkage is accompanied by the production of water molecule and this process is known as condensation.
- A dipeptide consists of two amino acids and one peptide bond. The dipeptide has an amino group at one end and a carboxyl group at the other end of the molecule.
- Therefore, both reactive parts are available for further peptide bonds to produce tripeptides, tetrapeptides, pentapeptides and so on leading to polypeptide chains.
STRUCTURAL ORGANIZATION OF PROTEINS:
Each protein has specific properties which are determined by the number and the specific sequence of amino acids in a molecule and upon the shape which the molecule assumes as the chain folds into its final, compact form.
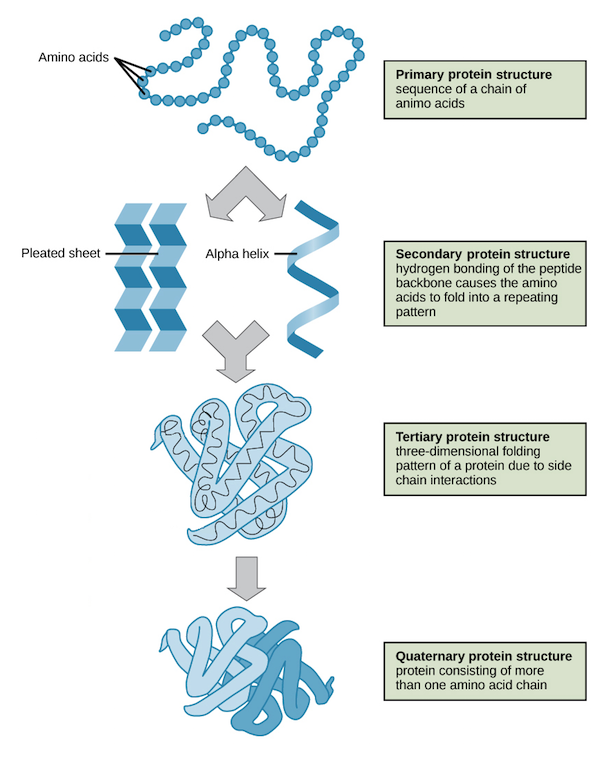
There are four levels of organization of proteins which are described below
PRIMARY STRUCTURE:
- The amino acids contained in a chain and the sequence in which they are joined is called the primary structure of proteins.
- Simply put, primary structure comprises the number and sequence of amino acids arranged in a linear sequence within a protein molecule.
DISCOVERY: F. Sanger was the first scientist, who in 1951 studied the primary structure of insulin and determined the sequence of amino acids in insulin.
EXAMPLES: Insulin is composed of 51 amino acids arranged into two chains. One of the chains has 21 amino acids and the other has 30 amino acids. The two chains are held together by disulphide bridges.
SECONDARY STRUCTURE:
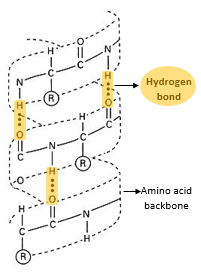
- Secondary structure is the structure of a protein molecule resulting from the regular coiling or folding of the chains of amino acids.
- This coiling or folding of the protein chain results from the formation of hydrogen bonds between the oxygen of the -CO- group of one amino acid and the hydrogen of the -NH- group of the amino acid four places ahead in the sequence.
TYPES OF SECONDARY STRUCTURE:
Secondary structure of proteins may be of two types
- α helix.
- β pleated sheet
α helix:
- the α helix is a tightly packed coiled formation of a polypeptide chain with the R-side chains of constituent amino acids extending outward from the central axis.
- The α helix structure is stabilized by extensive hydrogen bonding among the amino acids.
- The α helix is very uniform geometric structure with 3.6 amino acids in each turn of the helix
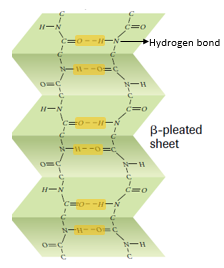
β pleated sheets:
- β pleated sheets are loose, much straighter configuration of protein polypeptide chains formed by the infolding of the polypeptide.
- In the β sheets, hydrogen bonds are formed between the neighbouring segments of polypeptide chain(s).
- β pleated sheets may be formed either by separate polypeptide chains or a single polypeptide chain folding back on itself
TERTIARY STRUCTURE:
- The compact structure of a protein molecule resulting from the three-dimensional coiling of the already folded chain of amino acids.
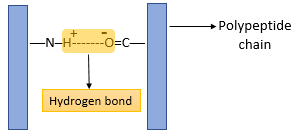
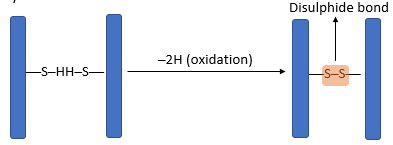
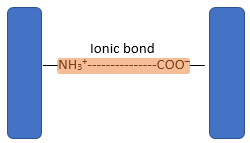
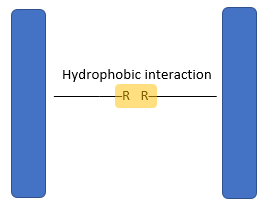
- The three-dimensional conformation of tertiary proteins is maintained by four types of bonds i.e. ionic bonds, hydrogen bonds, disulphide (-S-S) bonds and hydrophobic interactions.
- The hydrogen bond is formed between strongly polar groups such as –NH–, –CO– and –OH groups.
- Disulphide bonds are strong covalent bonds formed between cysteine molecules.
- Ionic bonds form between ionised amine (NH3+) groups and ionised carboxylic acid (COO─) groups.
- Weak hydrophilic interactions occur between non-polar R groups.
QUATERNARY STRUCTURE:
- A great majority of proteins are composed of single polypeptide chains. Some proteins, however, consist of two or more polypeptide chains giving rise to complex quaternary structures.
- Quaternary structure is held together by the same four types of bonds as in tertiary structure.
- The most salient example of quaternary structure is haemoglobin which is discussed in great detail below.
TYPES OF PROTEINS:
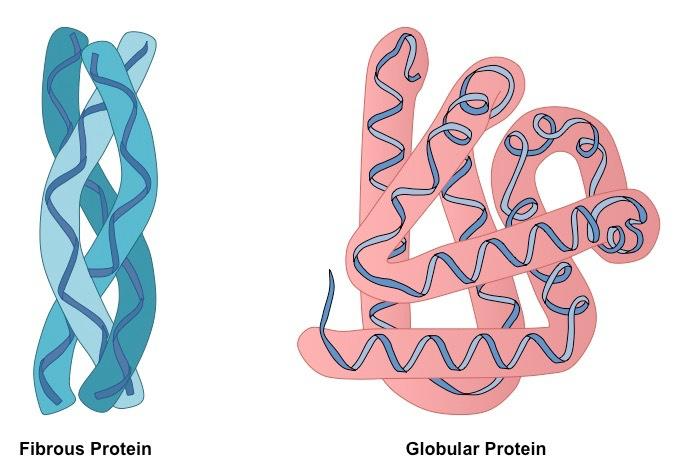
Based on shape, proteins may be of two types:
GLOBULAR PROTEINS:
- Globular proteins are spherical or ellipsoidal due to multiple folding of polypeptide chains.
- In living system, globular proteins are usually present in aqueous environments such as blood, tissue fluid, phloem in plants etc.
- Globular proteins curl up so that their non-polar, hydrophobic R groups point into the centre of the molecule away from the hydrophilic surrounding.
- Water molecules are excluded from the centre of folded protein molecule.
- The polar, hydrophilic R groups remain on the outside of the molecule.
- Globular proteins are therefore soluble in water because water molecules cluster around their outward pointing hydrophilic R groups.
EXAMPLES: Haemoglobin, Enzymes, hormones, antibodies.
FIBROUS PROTEINS:
- Fibrous proteins consist of molecules having one or more polypeptide chains arranged in the form of fibrils.
- Fibrous proteins are usually insoluble in water and have structural roles.
EXAMPLES:
- Keratin (hair, nails, hooves, horns)
- Myosin (muscle cells)
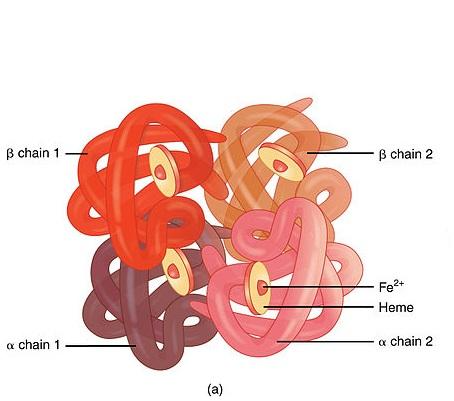
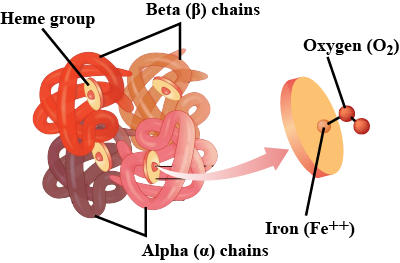
HAEMOGLOBIN-A GLOBULAR PROTEIN:
Haemoglobin is an oxygen carrying pigment found in red blood cells. It has a quaternary arrangement of polypeptides and is globular in shape.
GLOBULAR STRUCTURE:
- The haemoglobin is nearly spherical with the four polypeptides packed closely together.
- The hydrophobic R groups point towards the centre of the molecule and the hydrophilic ones pointing outwards.
- The interactions between hydrophobic R groups inside the molecule are important in holding haemoglobin in its correct three-dimensional shape.
- The outward pointing hydrophilic R groups on the surface of the molecule are important in maintaining its solubility.
FUNCTION OF HAEMOGLOBIN:
Haemoglobin act as a transport vehicle, shuttling oxygen from the lungs to all parts of the body.
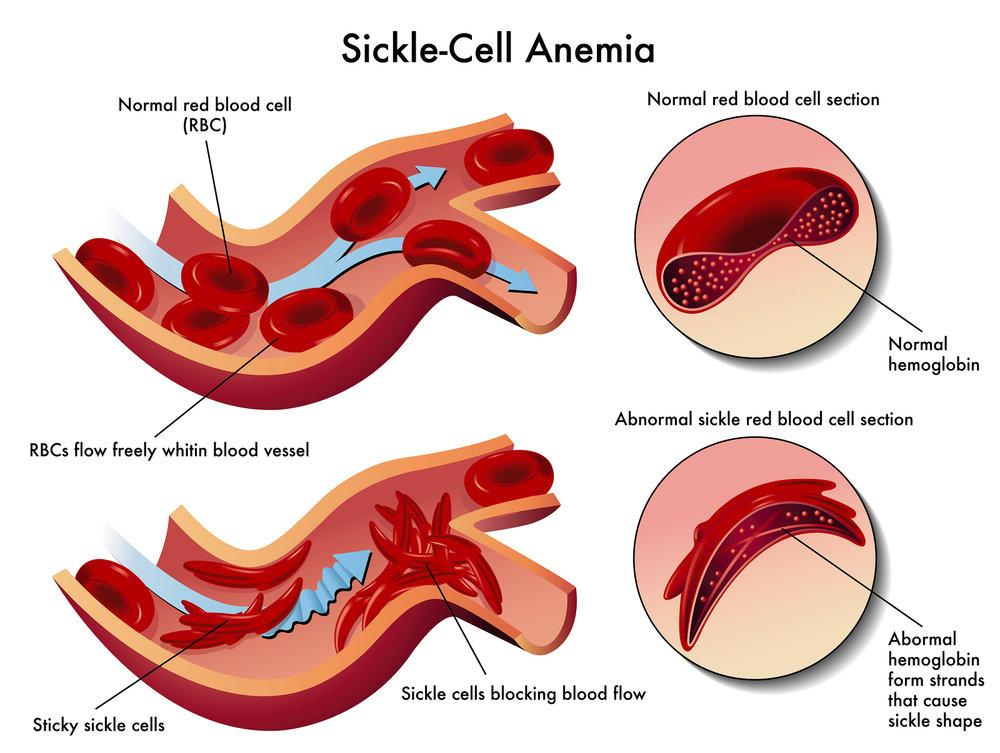
SICKLE CELL ANAEMIA:
- Sickle cell anaemia is a genetic disorder in which one amino acid occurring on the surface of β chain is replaced with a different amino acid.
- This replacement causes the alteration of normal globular structure of haemoglobin resulting in a sickle shaped haemoglobin molecule.
- The correct amino acid is glutamic acid (polar) which is replaced by valine (non-polar)
- Having one non-polar R group on the outside of the molecule makes the haemoglobin much less soluble and causes the unpleasant and dangerous symptoms of sickle cell anaemia.
IMPORTANCE OF PROTEINS:
- Proteins act as structural components in the body such as collagen in bones, keratin in hair and nails, myoglobin in muscles.
- Proteins are essential components of cell membranes.
- Protein plays an important role in various dynamic functions of the body such as;
- Hormones which are chemical compounds responsible for signalling the endocrine processes in the body.
- Haemoglobin, a quaternary protein, is responsible for the transport of oxygen within the body.
- Actin and myosin are responsible for the contraction and relaxation of muscles.
- Antibodies, which attack and destroy invading foreign substances, are proteins.
- Enzymes are involved in the catalysis of various anabolic and catabolic reactions.
- Proteins also act as storage products such as casein in milk and ovalbumin in egg white.

Responses